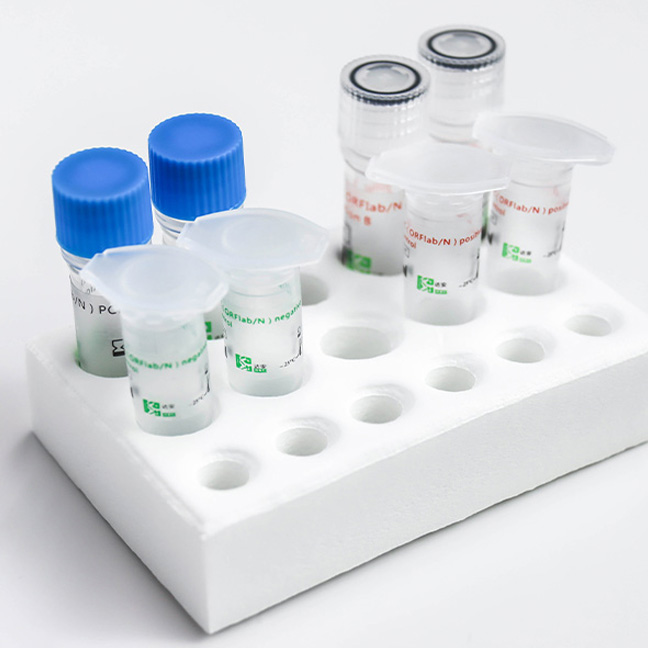
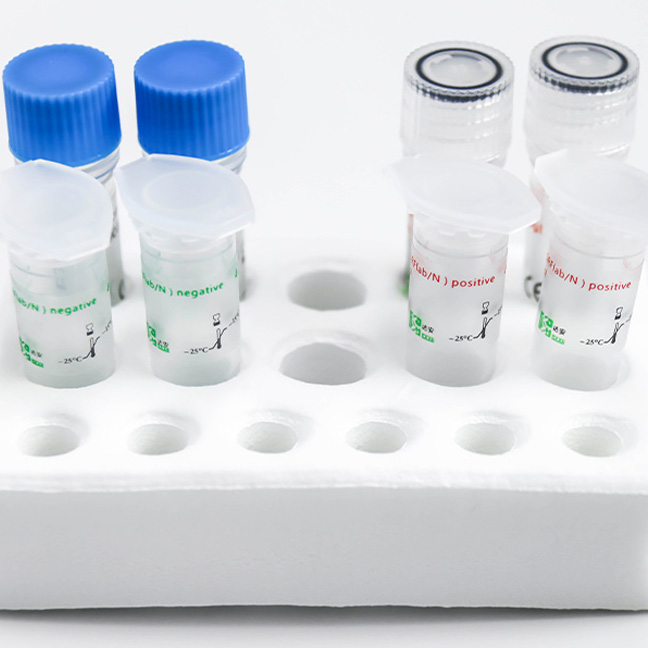
Detection Kit for 2019 Novel Coronavirus (2019-nCoV) RNA (PCR-Fluorescence Probing)
This covid-19 PCR test kit is used for in vitro qualitative detection of the ORF1ab and N genes of novel coronavirus (2019-nCoV) from throat swabs and sputum samples in pneumonia suspected cases of novel coronavirus infection, patients with suspected cluster cases, and other patients requiring diagnosis or differential diagnostic test for covid 19.