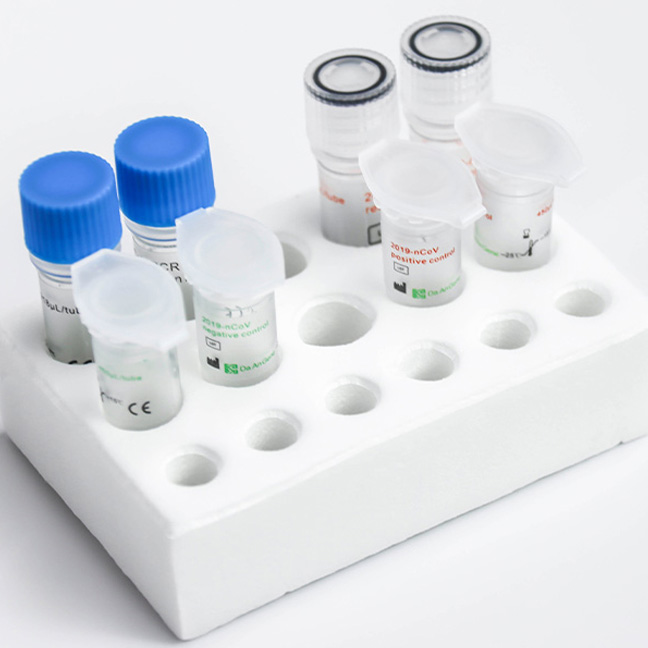
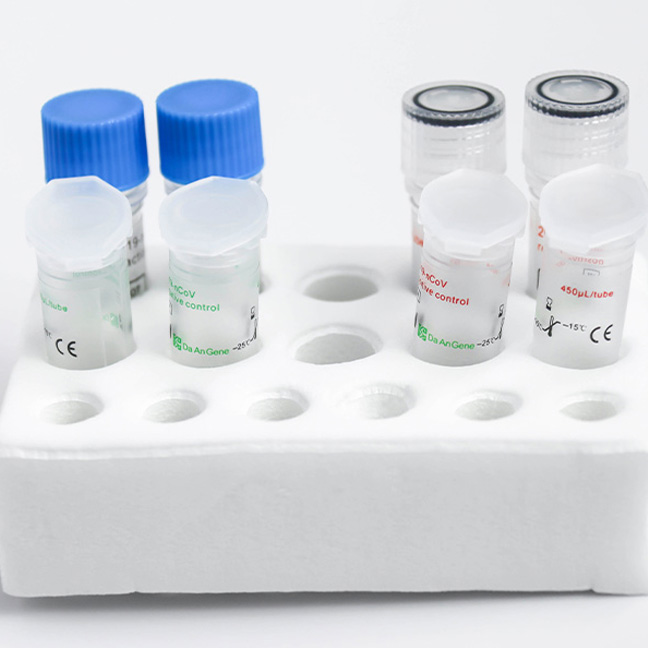
Detection Kit for 2019-nCoV (PCR-Fluorescence)
Respiratory infections are associated with various symptoms, including runny nose, congestion, body aches, cough, fatigue, sore throat, etc. Some respiratory infections are life-threatening, such as bacterial pneumonia and tuberculosis (TB). Daan Gene molecular diagnostic kits for respiratory PCR testing are designed for detecting the nucleic acid of respiratory infection could assist in the diagnosis of respiratory infection, and monitor the efficacy of the drug. Compared with the traditional methods, real-time fluorescent quantitative respiratory virus PCR kits like mycoplasma pneumoniae pcr test, are more accurate, rapid, and reliable.