Hepatitis B virus (HBV) and hepatitis C virus (HCV) infection are major causes of acute and chronic liver disease (e.g. cirrhosis and hepatocellular carcinoma) globally, and cause an estimated 1.4 million deaths annually.Currently, approximately 248 million people are living with chronic HBV infection, and 110 million persons are HCV-antibody positive, with 80 million have active viraemic infection. The burden of chronic HBV and HCV remains disproportionately high in low- and middle-income countries (LMICs), particularly in Asia and Africa. Additionally, even in low-prevalence areas, certain populations have high levels of HCV and HBV infection, such as persons who inject drugs (PWID), men who have sex with men (MSM), people with HIV, as well as those belonging to certain indigenous communities.
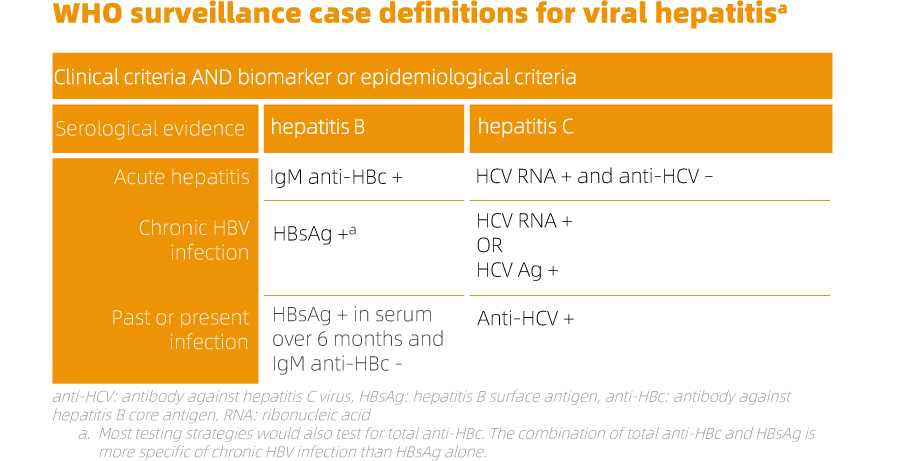
In the context of hepatitis virus infections, many individuals may not exhibit acute symptoms that go unnoticed by the healthcare system. Consequently, even capturing all diagnosed cases in clinical settings underestimates the actual number of new infections. Chronic infections can remain asymptomatic for decades, necessitating proactive measures like biomarker surveys to assess population prevalence. While research studies estimate the global disease burden of HBV and HCV infections, only a few surveillance systems consistently record the proportion of cirrhosis and/or HCC (hepatocellular carcinoma) attributable to these viruses. Testing and diagnosis serve as crucial gateways to prevention and treatment services, forming an integral part of an effective response to the hepatitis epidemic. Early identification allows individuals with chronic infections to receive necessary care and treatment, preventing or delaying liver disease progression. Testing also facilitates linking individuals to interventions that reduce transmission, including counseling on risk behaviors, provision of prevention commodities (such as sterile needles and syringes), and administering hepatitis B vaccination.
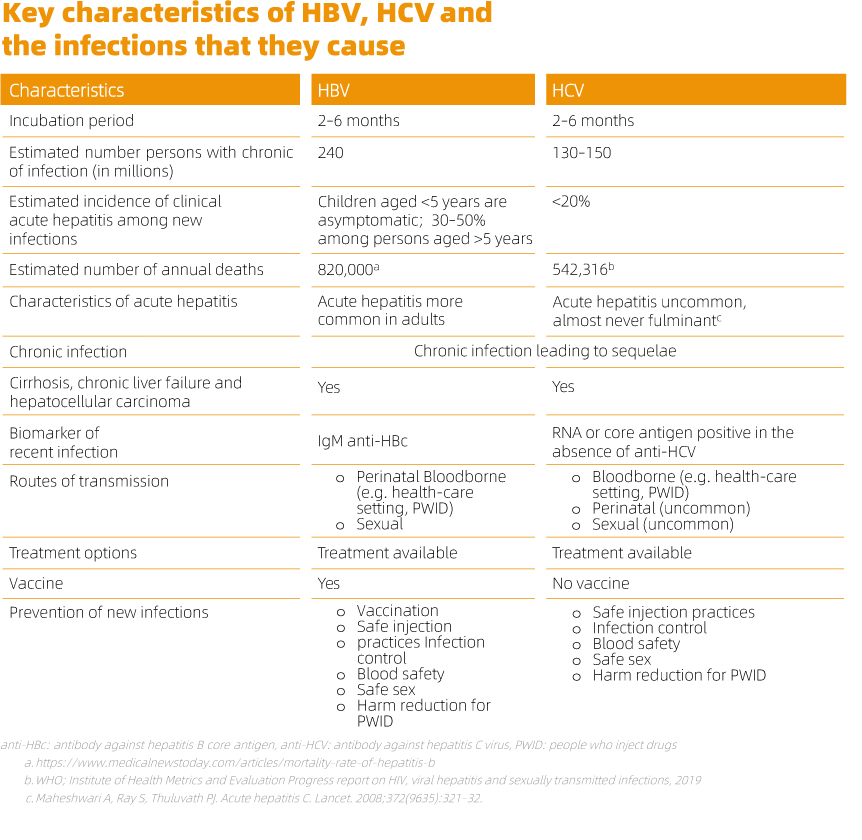
Viral hepatitis surveillance relies on in vitro diagnosis, primarily to identify the specific virus causing acute or chronic hepatitis and distinguish between recent, resolved, and chronic infections. The Guidelines on Hepatitis B and C Testing recommends the use of a serological in vitro diagnostic test or rapid diagnostic test to detect HBsAg and HCV antibody followed by NAT (nucleic acid testing) to verify and monitor disease.
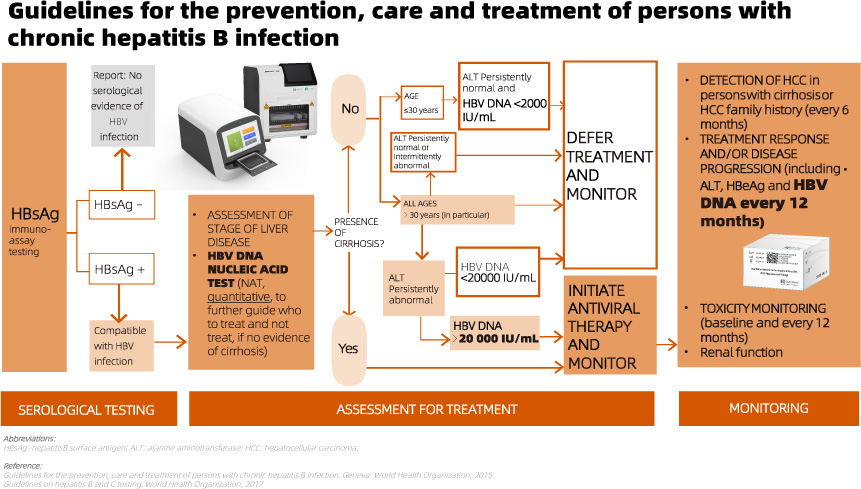
The recommendation is to use HBV DNA NAT following a reactive HBsAg serological test result. This aids in guiding treatment decisions, especially in cases without evidence of cirrhosis, and facilitates monitoring for treatment response.
As a expert of Viral Hepatitis detection, Daan provides an efficient real-time hepatitis b profile test kit. Daan's Hepatitis B (HBV) test kit employs advanced molecular techniques to amplify and analyze targeted regions of HBV DNA. This kit offers a dependable solution for conducting Hepatitis B DNA tests.
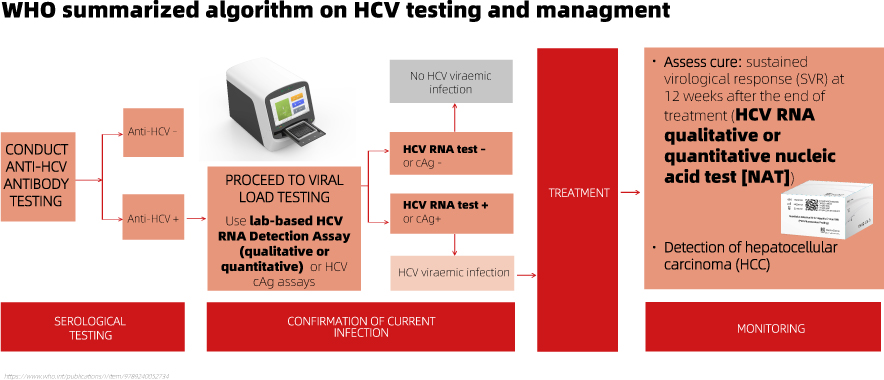
Following a reactive HCV antibody serological test result, a quantitative or qualitative RNA NAT is recommended as the preferred testing strategy to diagnose viraemic infection. Detection of the core HCV antigen may be considered as an alternative irrespective of the critical demands for sensitivity.
For the detection of HCV, Daan offers a HCV PCR kit. Utilizing advanced real-time RT-PCR technology, Daan Gene's HCV RNA Real-time PCR kit enables a rapid and accurate assessment of viral RNA levels, providing valuable information for healthcare professionals.
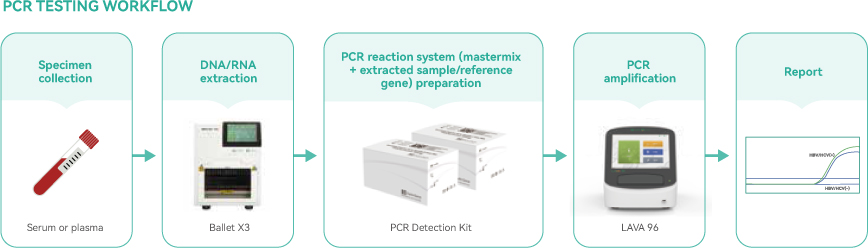
For clinical HBV and HCV diagnosis, high-quality sample preparation is the key factor to get downstream verifiable results. The serological sample (normally serum or plasma) should be purified and nucleic acid enriched before PCR amplification, increased sample volume capacity and enhanced extraction efficiency helps to reduce the burden of virus detection ability from a limited clinical sample.
Daan Gene recently launched a pair of ultrahigh sensitivity HBV and HCV PCR detection kit designed for individual testing and disease monitoring in strict compliance with WHO guidelines. This cutting-edge kit, boasting a remarkable limit of detection (LoD) of 2 IU/ml for Hepatitis B Virus and 10 IU/ml for Hepatitis C Virus, utilizes real-time PCR technology. Additionally, it incorporates UNG and dUTP to prevent contamination during experiments. With its superior sensitivity and unrivaled detection capabilities, this kit ensures the precise and accurate identification of hepatitis viruses.
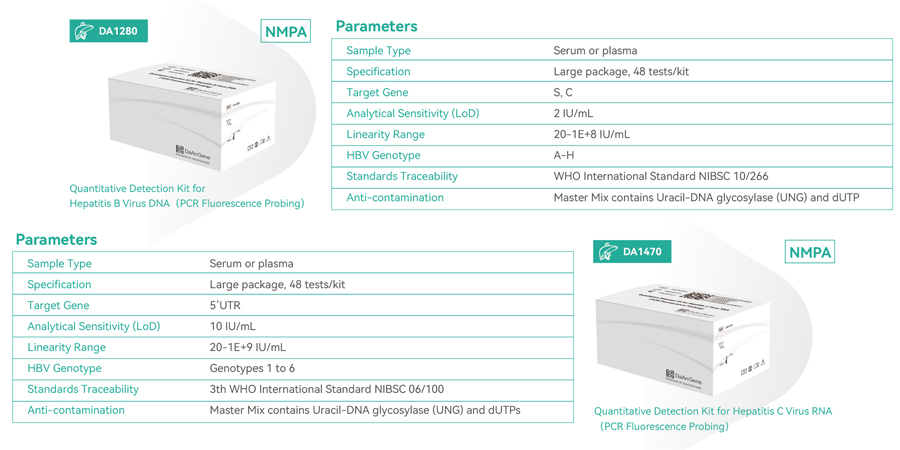
If you require our advanced PCR detection kits for Hepatitis B and C viruses, please feel free to reach out to our sales representatives or contact us via email at marketing@daangene.com. We are committed to providing precise and sensitive solutions for individual testing and disease monitoring.
https://www.who.int/publications/i/item/9789241549981
https://www.who.int/publications/i/item/9789241549059




