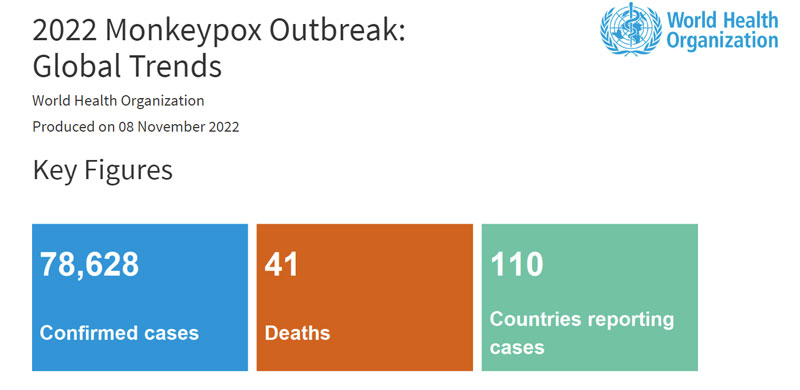
The WHO Director-General escalated the monkeypox outbreak as a Public Health Emergency of International Concern (PHEIC) in July. By November 8, there are 78,628 confirmed cases of monkeypox and the virus has spread across 110 countries, the monkeypox pandemic greatly aroused public concern around the world. At the beginning of the outbreak, there is a lack of effective tools to combat Monkeypox disease, including reliable and commercially available diagnostic kits. Mainstream diagnostic companies around the globe are sparing no effort to develop a monkeypox virus detection kit to stop the MPXV from spreading. As the first batch of companies to obtain the CE certificate on the monkeypox virus DNA detection kit, Daan Gene is always at the forefront of combating the outbreak of the monkeypox epidemic.
On October 19, 2022, the German Reference Laboratory for Poxviruses from Robert Koch Institute associated with the World Health Organization, Department of Epidemic and Pandemic Preparedness and Prevention evaluated the performance of 11 commercial PCR kits for Monkeypox virus DNA detection. The evaluation tests are based on WHO’s guidance - Laboratory Testing for the Monkeypox Virus, using the Polymerase chain reaction (PCR) method to detect the MPXV.

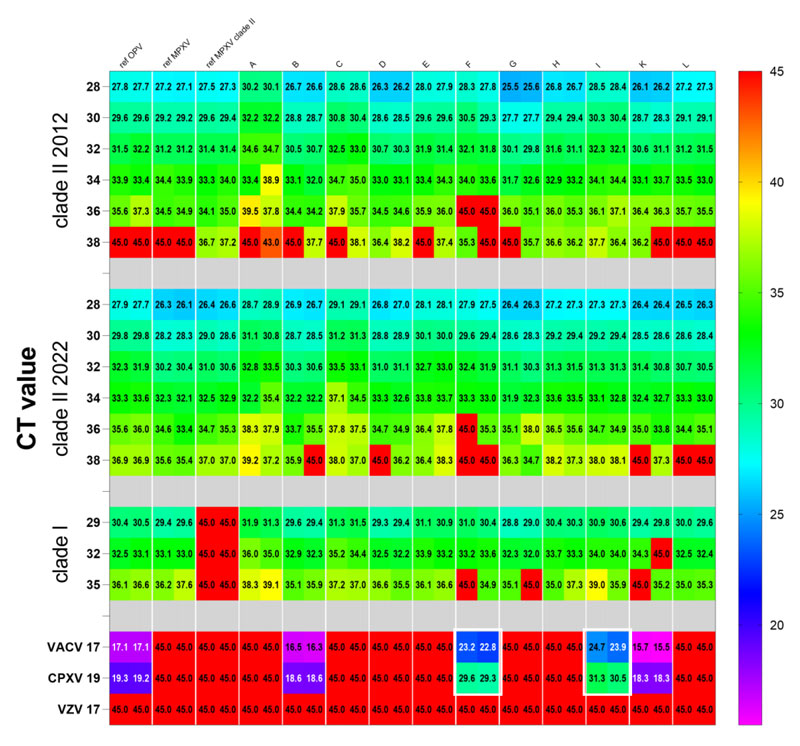
Strict control variable was introduced in the evaluation on 11 kits(A to L), and the results were compared to the reference diagnostic workflow including generic OPV PCR, MPXV-specific PCR, and MPXV clade II specific PCR. The experiment established an 18-specimen panel, including DNA from MPXV clade I, clade Ⅱa and clade Ⅱb, other OPV, and VZV. All samples were analyzed in duplicate. In addition, to ensure the result comparability among 11 various PCR kits, all kits were run on BioRad CFX 96 real-time cycler and the evaluation was based on the manufacturer’s manual to get the lowest CT value as low as possible.
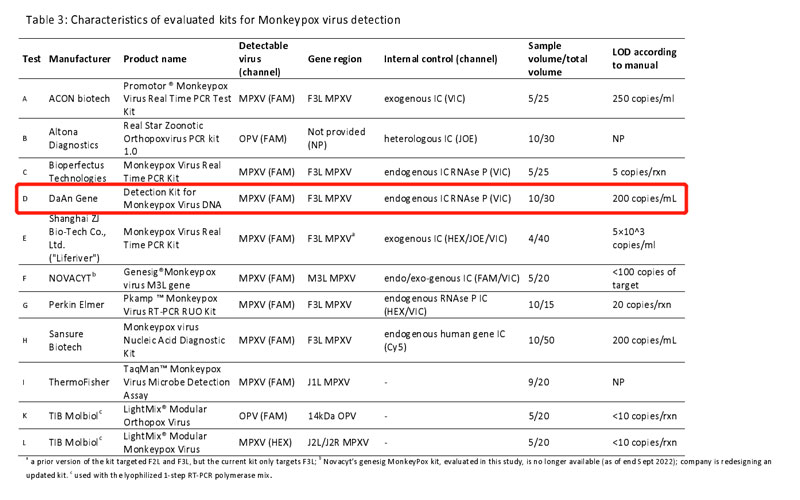
The evaluation result shows that 11 kits are with comparable high sensitivity and specificity to detect clade I and clade II monkeypox virus DNA. From a clinical diagnosis perspective, properly-sampled skin lesions are an ideal specimen for monkeypox virus detection for it is suitable to identify a range of clinically-relevant viral loads. In the WHO evaluation result, the Daan Gene monkeypox PCR kit performs relatively high sensitivity and specificity among other monkeypox diagnosis test kits.
Daan Gene's Monkeypox Virus Detection Kit complied with WHO recommended diagnosis method, nucleic acid amplification testing (NAAT). Currently, Polymerase chain reaction (PCR) is the gold standard for monkeypox virus infection diagnosis.

Reliable: Based on WHO recommended diagnosis method: real-time polymerase chain reaction (PCR)
High Specificity: Highly conservative region of Monkeypox virus gene coding region
High Accuracy: Involved endogenous internal to monitor the whole NAAT procedure from sampling to PCR result
High Compatibility: Compatible with a mainstream real-time fluorescent PCR system
>> Learn More about DaAn Gene Monkeypox Laboratory Diagnosis Solution
As a leading In-vitro Diagnostic(IVD) company with nearly 30 years of expertise in molecular diagnosis, Daan Gene's innovative diagnostic solution has helped millions of patients all over the world. We aim to increase the monkeypox diagnostic efficiency and provide a high-quality diagnostic product to combat monkeypox, we have launched a comprehensive solution on monkeypox diagnosis, check out Daan Gene’s White Paper on Monkeypox testing to see how DaAn Gene’s product advancing your monkeypox virus detection efforts.

>> Download Monkeypox Diagnosis White Paper Now
Reference:


