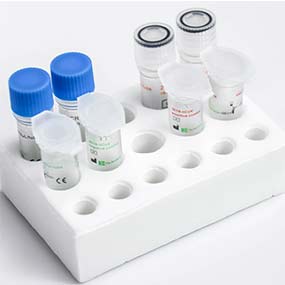
Detection Kit for Influenza A/B and 2019-nCoV (PCR-Fluorescence Probing)
This kit is designed for the identification of Influenza A virus, Influenza B virus, and 2019-nCoV in respiratory samples. With high specificity, sensitivity and repeatability, this kit is able to assist the diagnosis of Flu A/Flu B and 2019-nCoV.