Digestive infections are caused by some viruses, bacteria, or parasites leading to digestive organ inflammation, some of which can be fatal. The digestive system, including the gastrointestinal tract, liver, pancreas, and gallbladder plays an important role in digesting food in the human body. Digestive infections are usually characterized by abdominal cramps followed by diarrhea. Other symptoms might include vomiting, fever, nausea, decreased appetite, muscle aches, dehydration, bloody mucus in the stool, etc.
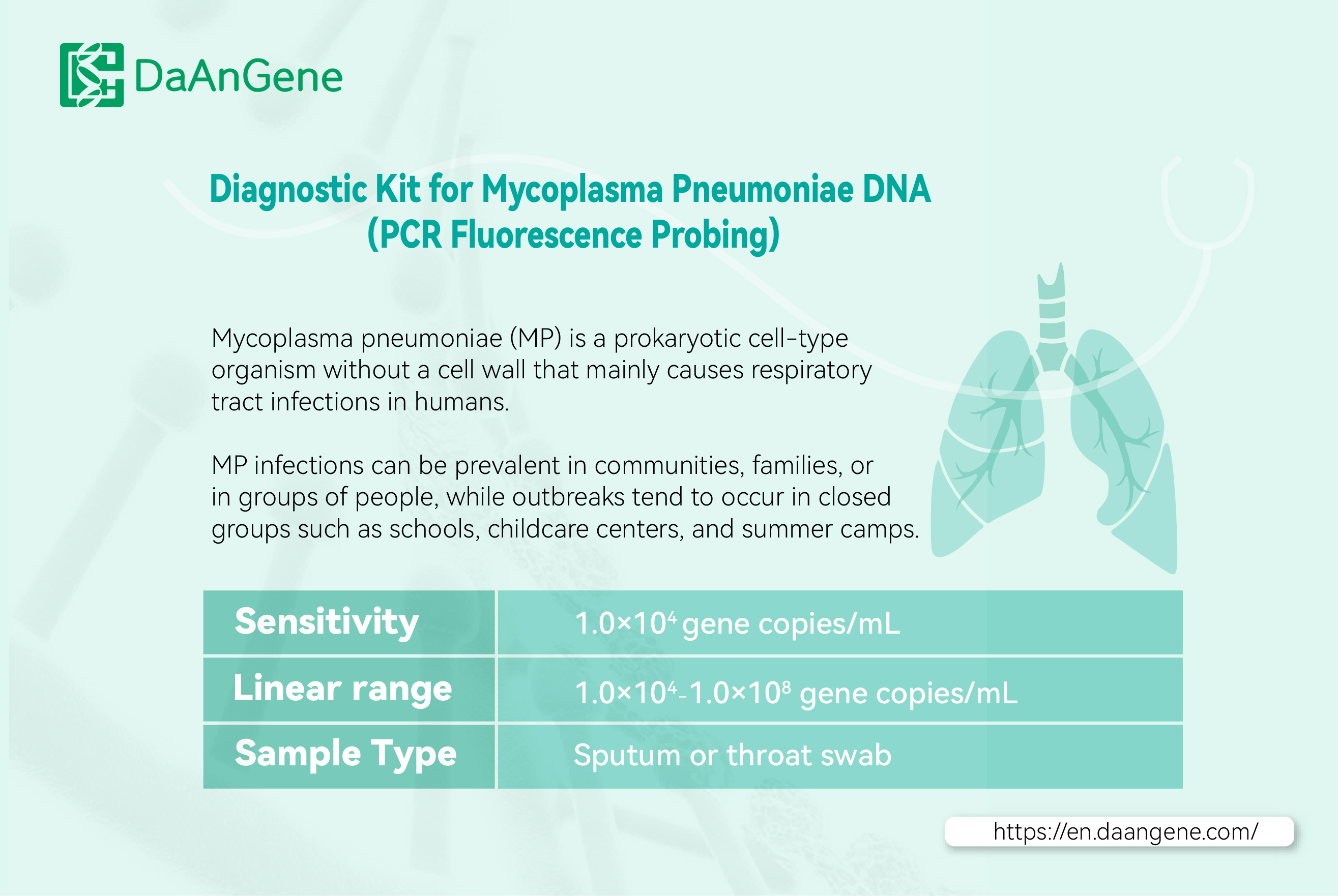
When suspicious symptoms of digestive infection appear, diagnosis can be confirmed through stool specimens or oral secretion samples with digestive testing kit. Therefore, Daan Gene's digestive test kit, including norovirus test kit and hand foot and mouth diagnostic test kit are uses real-time fluorescent PCR technology to detect human DNA and RNA, applicable to the auxiliary diagnosis of digestive infection.