Hepatitis C is a significant public health threat. According to the World Health Organization HCV prevalence statistics, approximately 58 million people were infected with the hepatitis C virus, and 1.5 million new infections every year globally. HBV infection is classified into several stages based on the level of liver damage. New HCV infections are mostly asymptomatic, so it is recommended to test HCV RNA level by NAAT. What’s more, during the antiviral therapy hepatitis c virus level should measure regularly to evaluate the efficacy of treatment.
Yuting He's team in the Department of Laboratory Medicine, The First Affiliated Hospital, Sun Yat‐sen University conducted a comparative evaluation of the HCV RNA quantitative assay performance between Daan Gene and the Roche Cobas. The evaluation complied with WHO International Standard for hepatitis C virus RNA for nucleic acid amplification techniques, to measure the performance of the HCV RNA quantitative assay.
The evaluation method is based on the WHO HCV RNA standard, NIBSC 06/102 standard, and CLSI EP documents to measure the diagnostic precision, linearity, accuracy, cross-reactivity, and anti-interference ability of the Daan Gene HCV RNA quantitative diagnostic kit. To compare the performance of two HCV RNA quantitative assays, 198 clinical serum specimens were used to evaluate the competence between Daan HCV RNA quantitative assay and the Roche Cobas test.
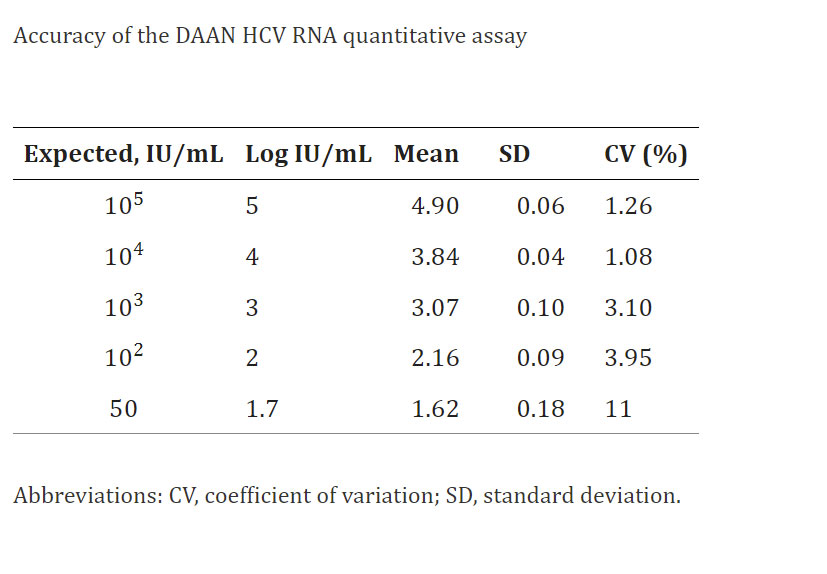
Precision and Accuracy: Different concentrations of HCV RNA were calculated in the study. The SDs and CVs for different concentrations of HCV RNA were shown in the below table(Accuracy of the DAAN HCV RNA quantitative assay). The Daan Gene HCV RNA test kit’s CVs of each concentration indicated excellent accuracy.
Linearity and Limit of Detection (LOD): Daan HCV RNA quantitative assay exhibited a linear response from 1.3 log IU/mL to 8 log IU/mL. The HCV RNA detection rates for nominal HCV RNA concentrations of 50, 20, 15, and 10 IU/mL were 100%, 100%, 100%, and 84%, respectively, Daan Gene HCV RNA detection kit performing high sensitivity.
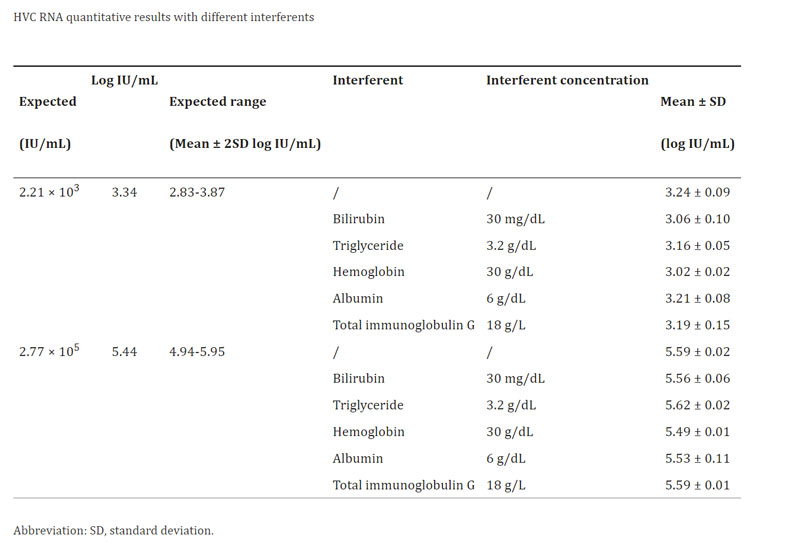
Interference and cross‐reactivity study: the cross-reaction study introduced different interfering substances such as bilirubin, triglyceride, hemoglobin, albumin, and total immunoglobulin G. The result showed that different interfering substances did not influence the accurate detection of high-concentration or low concentration HCV RNA‐positive serum and all test results were in the expected range. The Daan HCV RNA quantitative assay had not detected HCV RNA in the specimens positive for HBV, CMV, EBV, or DV. Thus, Daan HCV RNA quantitative assay showed no cross‐reactivity with HBV, CMV, EBV, and DV infections.[1]
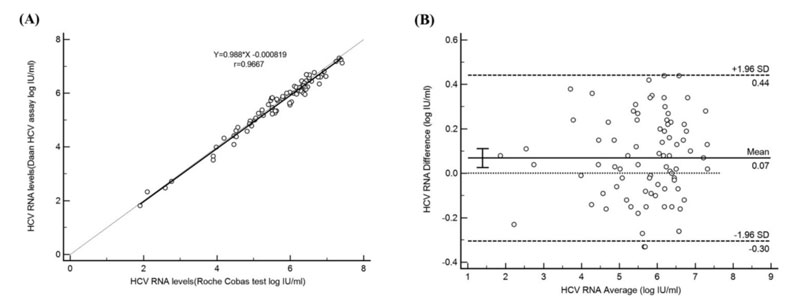
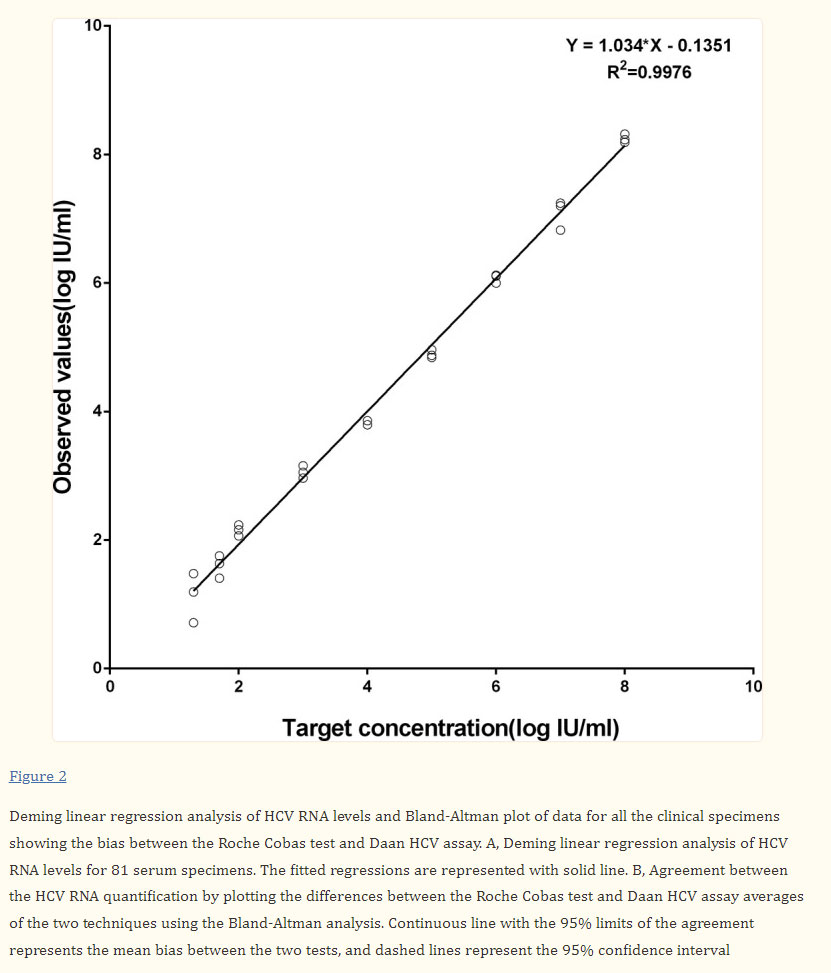
HCV RNA quantitative Assay Agreement Test between Daan Gene and COBAS AmpliPrep/COBAS TaqMan: Comparable performance of quantitative detection was illustrated by Deming regression analysis and Bland‐Altman analysis. A good correlation and agreement were observed between the two assays.[1]
The comparative evaluation study proved that Daan HCV RNA quantitative assay has excellent performance regarding precision, accuracy, linearity, anti-interference ability, and good agreement with the Roche assay. The test is based on the clinical specimen, which indicated Daan Gene HCV assay has good clinical performance and it is an idea candidate for hepatitis C diagnosis as well as monitoring the therapeutic efficacy of HCV infection patients. Moreover, Daan HCV RNA quantitative assays cost about a quarter less than Roche Cobas equivalents, which made it affordable in many developing countries.
After a series of comparative evaluations, the research proved that Daan Gene HCV RNA quantitative test kit with the merits of inexpensive and excellent analytical and clinical performance, which is a good choice for the diagnosis of HCV infection and monitoring HCV RNA levels in hepatitis c patients.
Daan Gene is the first Chinese company to obtain the CE List A approval for the HBV/HCV PCR diagnostic kit. As the world-leading hepatitis PCR detection kit provider, Daan Gene utilizes its expertise to promote hepatitis care. Accurate diagnosis is important in hepatitis treatment, Daan Gene dedicates to providing reliable and efficient solutions for viral hepatitis diagnosis.
Feature of DaAn Gene HCV RNA PCR Qualitative Test Kit
Easy and fast: Simple experimental operation, results are available within two hours.
Reliable results: No cross-reactivity with other pathogens.
High sensitivity: LoD 20 IU/mL
Internal control:Monitor the whole process.
>> Learn More about DaAn Gene HCV PCR Test Kit
Reference:
1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7370725/
2. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c



