Recently researchers from DaAn Gene Research Institute, the Medicine and Biological Engineering Technology Research Center of the Ministry of Health, Guangzhou, China and Clinical and Translational Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK developed a multiplex real-time RT-PCR assay to simultaneously monitor the infection of 9 pathogens of clinical human respiratory tract specimen. This innovative research was published on 03 August 2022 at Nature-Scientific Reports.

The novelty of this multiplex PCR assay simultaneously detects 9 kinds of pathogens by dividing them 3 by 3 into 3 tubes and in each tube parallelly detect the GAPDH gene under the circumstance of a 4-channels-equipped fluorescent PCR machine. Narrowed the turnaround time to 3.0 hours and the cost of assay around $20, reduced the waiting time and financial load for patients.
Many factors delayed the respiratory infected patients to get 100% appropriate treatment, such as neglecting the co-infection of more than 2 kinds of pathogenic microorganisms, an erroneous judgment of pathogenesis, improper medication, or patients’ noncooperation due to torment or poverty. This research aimed 9 pathogens influenza A and B viruses, adenovirus, RSV, Streptococcus pneumoniae, Legionella pneumophila, Haemophilus infuenzae, Chlamydia pneumoniae, and Mycoplasma pneumoniae, which are very common atypical pneumonia pathogenesis. The compatible 4 fluorescence channels are the most commonly used which is the regular configuration of most mainstream real-time PCR instruments. Simplified one-step PCR technology is friendly to the frontline clinics' diagnosis and epidemiological containment of acute respiratory infections.
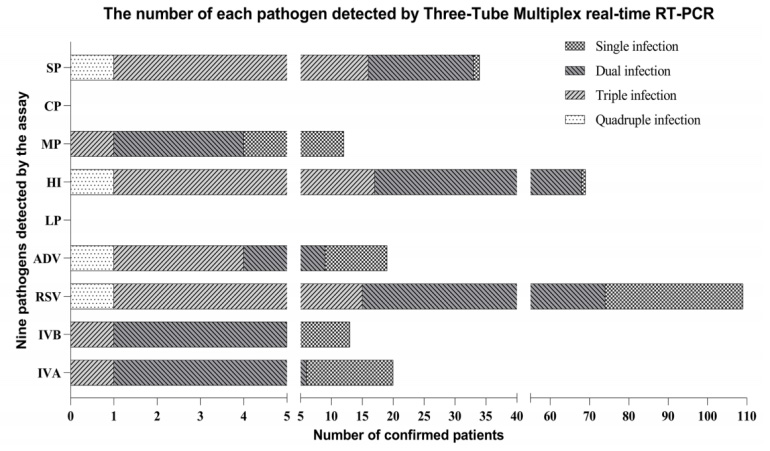
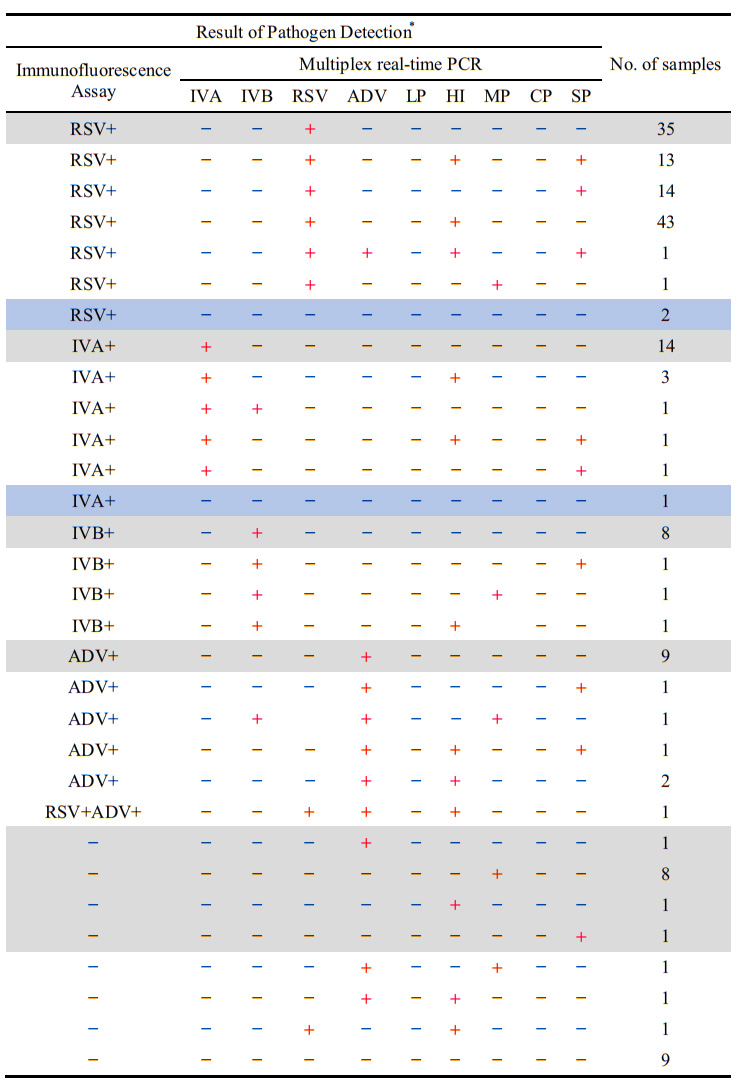
Figure 1: The results revealed that virus-bacteria co-infections were found in 90 (50.28%) patients who are mostly previously been diagnosed as single-infection. Co-infection is a tough and subtle problem for clinic diagnosis by conventional method, using multiplex PCR assay helps a lot.
Bacterial/viral cultures are the current gold standard for the diagnosis of acute respiratory infections, but it will take many days of waiting until the pathogen forms an identifiable clone to be identified. Another common-used method is the serological test, like the lateral flow applied COVID-19 test kit, chemiluminescent immunoassay or ELISA, they are as quite fast as about 15 min to get the result. But the immunoreaction-based principle limits the detection targets on the spike-protein of virus or the antibody stimulated by bacteria, cross-reaction with other similar pathogens results in false positives and confuses the clinical practitioner.
Both of the above methods doing weak in dealing with multiple pathogens-involved cases while polymerase chain reaction (PCR) doesn't. Regarding this, innovation combining high sensitivity and specificity, low cost, operational simplicity and practicability is in urgent need. Daan Gene will continue working on developing promising alternative assays to push the progress of the approaches used for early screening for public disease.
DOI



