Cervical Cancer Awareness Month is aimed to promote awareness of cervical cancer prevention, diagnosis, and treatment. Cervical cancer is one of the most common health problems in women. According to World Health Organization statistical results, there are nearly 604,000 females diagnosed with cervical cancer and 342,000 deaths of cervical cancer worldwide in 2020.
Cervical cancer is the malignant cells grow out of control in the cervix, it usually occurs near the cervical orifice at the lower end of the cervix. Cervical cancer is mainly caused by long-term infection with high-risk human papillomavirus (HPV) with a relatively long precancerous lesion process. HPV is primarily transmitted through sexual contact, about 60-80% of women who have sexual activity would be infected with at least one type of HPV in their lifetime. Intervene and treat at the stage of precancerous lesions (cervical intraepithelial neoplasia, CIN) or the early stage of cancer, cervical cancer is curable. Therefore, screening tests play an important role in preventing and treating cervical cancer.
World Health Organization officially launched the Global Strategy for Elimination of Cervical Cancer as a Public Health Problem by 2030. Declare that the world will achieve the "90-70-90" goal by 2030 and eliminate cervical cancer by the end of this century.
Vaccination: 90% of girls receive full course of HPV vaccine by the age of 15
Screening: 70% of women have high-performance cervical cancer screening test by the age of 35 years, and the second screening test before 45 years old
Treatment: 90% treatment rate for precancer in women and 90% of women get invasive cancer management
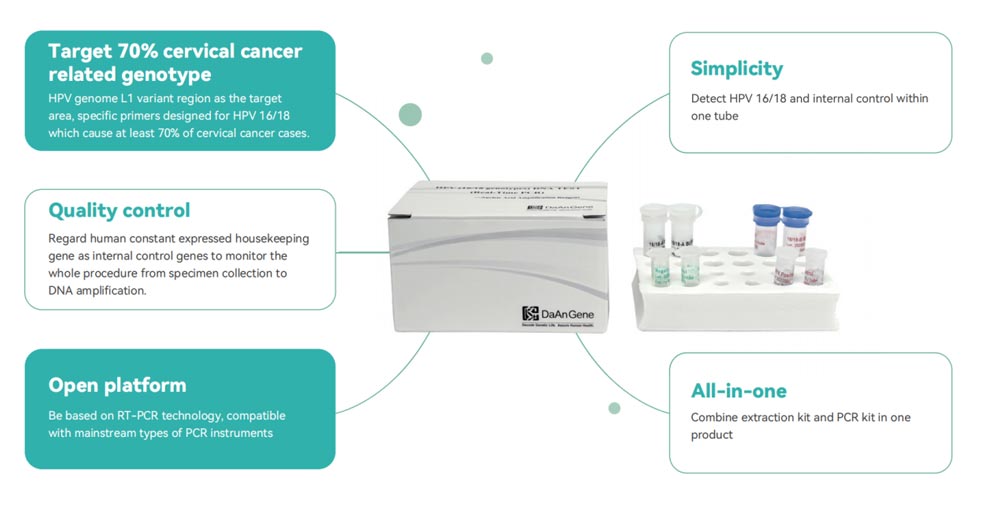
Human Papillomavirus (HPV) PCR Test
WHO recommend HPV DNA testing as the first-choice diagnostic method for cervical cancer prevention. Daan Gene HPV (16/18 genotypes) DNA Test (Real-Time PCR) is used to qualitatively detect human papillomavirus (HPV) type 16 and type 18 deoxyribonucleic acid (DNA) in cervical exfoliated cells and genitourinary secretions specimens.
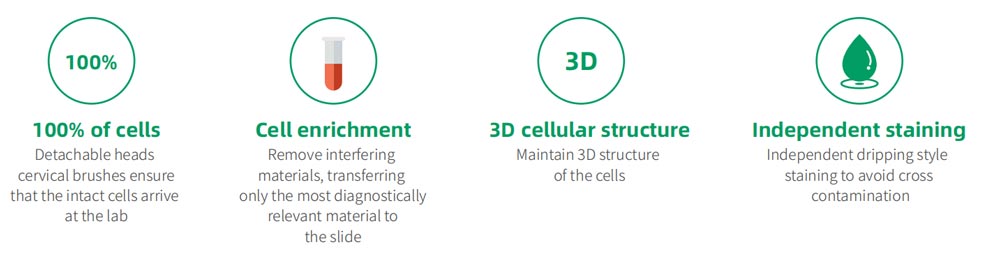
Liquid Based Cytology System for Cervical Screening
The LBP automatic sample processor is designed for the pre-processing of cervical cells and LBP-2264C Liquid-based Cytology Production Machine is a liquid-based cytology specimen collection, preservation, and automated tissue processor that produces high fidelity single layer cell slides with the specimens of cervical cells and other kinds of specimens.
Reference
https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
https://www.who.int/europe/news/item/11-09-2021-who-recommends-dna-testing-as-a-first-choice-screening-method-for-cervical-cancer-prevention




