According to CDC, the US is in a flu epidemic now. Facing a “triple epidemic” of COVID-19, influenza, and respiratory syncytial virus (RSV) simultaneously. CDC estimates there have been at least 1 600 000 illnesses, 13 000 hospital admissions, and 730 deaths from flu. The medical and health system is facing huge pressure due to the superposition of the “triple epidemic” situation. For COVID-19, influenza and respiratory syncytial virus (RSV) share similar symptoms, such as fever, fatigue, and cough, which makes it difficult to identify what pathogen causing the illness. The co-testing approach helps increase testing capacity during the busy flu season and speeds up the time to a diagnosis.
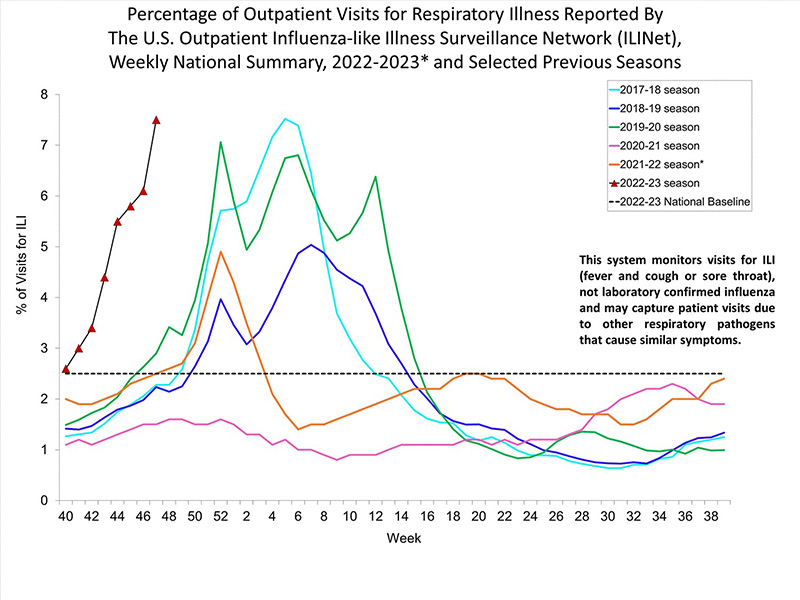
Source: https://www.cdc.gov/flu/weekly/index.htm#ILINet
Daan Gene cov-2 flu a+b test kit is based on a one-step RT-PCR technique, which can realize the auxiliary differential diagnosis of three kinds of virus infection. Detailed performance studies confirm the high specificity, sensitivity and repeatability of this kit, which can assist in the diagnosis of Influenza A virus, Influenza B virus and 2019-nCoV infection. By eliminating multiple tests or doctor visits, the patient can get an accurate treatment plan as soon as possible. The co-testing approach helps accelerate the testing capacity and efficiency during the busy flu season.
Influenza A/B and 2019-nCoV Test Kit Features
Test for Flu A/B and 2019-nCoV with one sample, providing solution for management of influenza-like illness patients.
Simultaneous detection of two different target genes of 2019-nCoV.
Endogenous internal control: human housekeeping gene RNase P.
High specificity, no cross-reaction with other similar pathogens.
>> Learn More about Daan Gene COVID and Flu Kit
Reference:
1. https://www.bmj.com/content/379/bmj.o2681
2. https://www.cdc.gov/flu/weekly/index.htm#ILINet