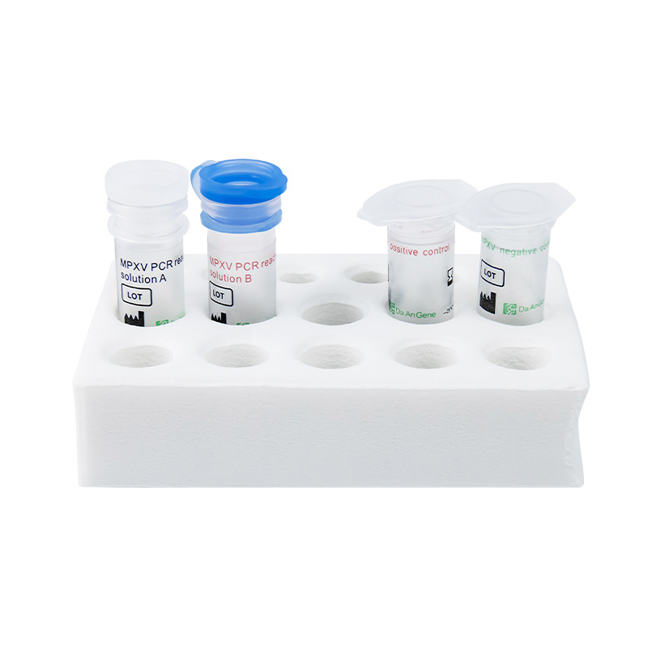
Detection Kit for Monkeypox Virus DNA (PCR-Fluorescence Probing)
Daan Gene's Monkeypox test kits are specialized diagnostic tools designed for the detection and identification of the monkeypox virus, an orthopoxvirus that can cause a rare but potentially serious viral disease in humans. Polymerase chain reaction (PCR) is the preferred laboratory test given its accuracy and sensitivity which suggested by WHO. Confirmation of PCR test for monkeypox virus infection is based on nucleic acid amplification testing (NAAT), using real-time polymerase chain reaction (RT-PCR). The preference is often given to specialized tools such, as the monkeypox detection kit, which is designed for detecting distinctive sequences of viral DNA.
This Monkeypox Virus PCR test kit can be utilized independently for accurate and specific detection.