To eliminate viral hepatitis, it is necessary to improve the public's awareness of viral hepatitis and increase the diagnosis and treatment efficiency of viral hepatitis. Advanced viral hepatitis diagnostic assays are essential to tackle HBV and HCV.
Hepatitis B is liver inflammation caused by the hepatitis B virus(HBV). Hepatitis B antiviral therapy is aiming to inhibit the replication of the hepatitis B virus and reduce the HBV DNA copies level, to decrease the risk of liver cancer. High-sensitivity HBV DNA diagnostic assays are of great importance in clinical diagnosing. Currently, improving criteria of HBV DNA PCR diagnostic assay from different hepatitis academic association reflects the rising attention towards eliminating HBV from all over the world.
Guidelines | Sensitivity | |
2015 | Guidelines of Asia Pacific Society of Hepatology (APASL) | ≤12 IU/ml |
Guidelines of World Health Organization (WHO) Hepatitis B | ≤15 IU/ml | |
2017 | The European Society of Hepatology (EASL) | The lower the better, Recommend ≤10 IU/ml |
2018 | Guidelines of American Academy of Hepatology (AASLD) | ≤10 IU/ml |
An investigation conducted by Wang Yajie's team at Beijing Ditan Hospital affiliated with Capital Medical University collected plasma from 514 outpatients and inpatients with hepatitis B who visited the hospital from February to June 2019. Professor Wang's team conduct parallel testing on the Daan Gene high-sensitivity HBV virus nucleic acid quantitative testing kit and other international mainstream HBV detection assays, to test the viral load in the plasma of hepatitis B patients and then conducted a comparative analysis of the correlation and consistency of the two testing results.
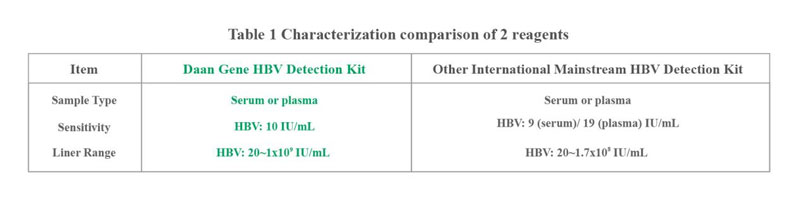
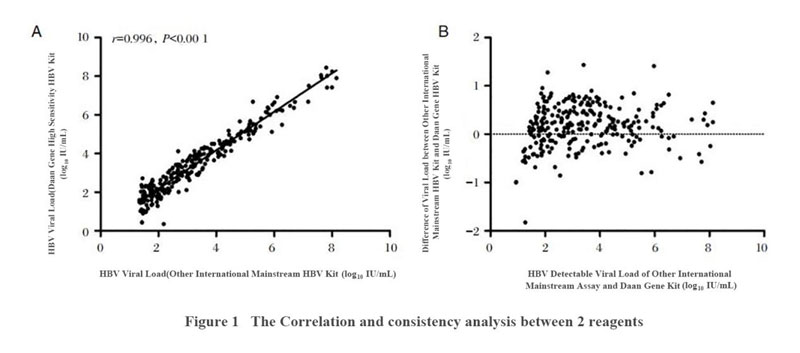
The results prove that Daan Gene's high-sensitivity HBV reagent increased the linear range to 109 IU/ml, and reduced the detection limit to 10 IU/ml. The detection result shows that the effective values of the two reagents had good correlation and consistency.
HBV tends to mutate quite more easily than other DNA virus due to the unstable structure of the HBV genome. The mutation of HBV changes the biological behavior of the virus and decrease the efficacy of antiviral drugs, affecting the therapeutic effect and the progress of the disease. Hepatitis b virus DNA quantitative test can help identify occult HBV infection (OBI) and occult chronic hepatitis B. In addition, high sensitivity test assay of HBV DNA is crucial for the diagnosis of serologically atypical chronic HBV infection, and it is of great significance for the confirmation of inactive HBsAg carrying status (serum HBV DNA<2000IU/ml).
High-sensitivity HBV detection assay not only can screen the occult HBV infection, but also serve as an important indicator for preoperative examination, risk assessment of HBV reactivation after radiotherapy and chemotherapy in tumor patients, efficacy and end-point evaluation of antiviral therapy, and prediction of HBV resistance mutations in HBeAg-negative patients.
In the Daan Gene HBV DNA Quantitative PCR assay, a specific primer and a fluorescence probe are designed by adopting the fluorescence PCR technology and selecting a relatively conserved region in the HBV genome as a target region. After the sample nucleic acid is purified, the HBV DNA is quickly quantitatively detected by PCR. In addition, the Daan Gene HBV test kit also contains an internal standard substance for monitoring the whole process of nucleic acid extraction, to reduce false-negative results.
Features of DaAn Gene HBV Test Kit
Wide liner range: 20 IU/mL-1.0×109 IU/mL.
Internal control: monitor the whole extraction process.
Reliable: UNG enzyme to prevent contamination.
High Sensitivity: LOD 10 IU/mL
Genotype: Cover HBV genotype A-G
>> Learn More about DaAn Gene Hepatitis B Test Kit
Reference:
J Mol Diagn Ther, September 2019, Vol. 11 No. 5