The detection methods of Sars-Cov-2 can categorize into two types according to different detection objects and techniques. Molecular biological testing and immunological testing are commonly used methods in diagnosing COVID-19 infections. The nucleic acid amplification test(NAAT) is the “golden standard” for clinical diagnosis of COVID-19, which belongs to molecular biological diagnosis, and immunological testing including rapid antigen detection and antibody detection.
Some factors may interfere with the nucleic acid test results, so "false positives" and "false negatives" may occur.
① Contamination of samples due to human error;
② Technical limitation, e. g. the antigen test is more probable to occur false positive;
③ Cross-contamination with other pathogens due to the design of primers or other components.
① Inappropriate sample collection, transportation, storage and processing, and low pathogen concentration in the sample;
② Failed to collect the signal of the indicator from the testing device;
③ Other possible interference that has not been verified.
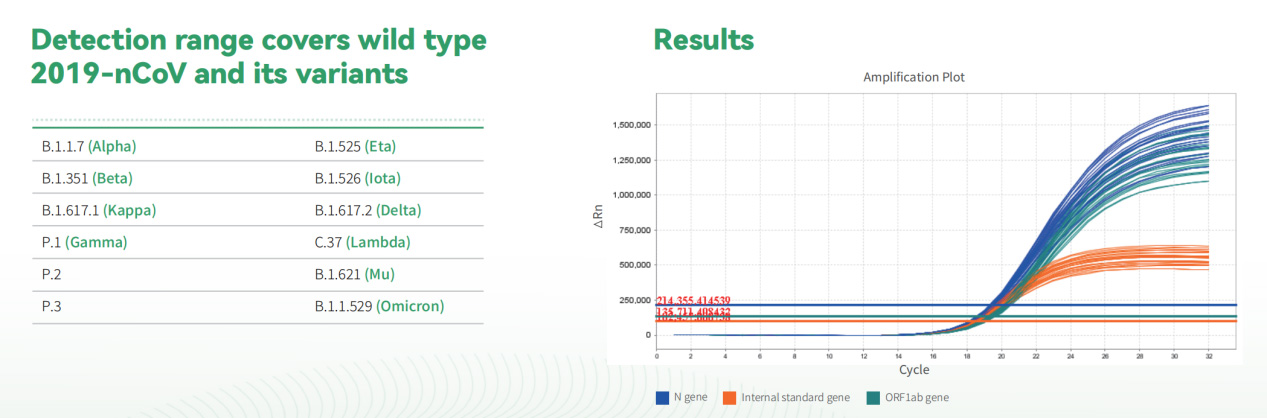
The mutation of the novel coronavirus strain mainly concentrated on the S gene, so some antigen test regards the S gene of Sars-Cov-2 as the target gene while the PCR targets a relatively unique and conservative sequence, such as the ORF1ab gene and N gene, which are barely mutable. If people infected by the mutant strain, it can still be detected through the nucleic acid amplification test like PCR and sequencing. Therefore, Sars-Cov-2 PCR testing is the primary method of COVID-19 diagnosis despite the virus constantly mutating.
CT value mainly refers to the number of PCR amplification cycles when the fluorescence signal reaches the threshold value. For example, if the virus load in our samples is relatively low, and it will take more times of amplification to reach the threshold so the CT value is larger than the CT cut-off. While the virus load concentration is relatively high, the CT value is relatively smaller than the CT cut-off.
SARS-CoV-2 Omicron variant shares similar symptoms to the previous variants but with faster transmission speed and stronger infectivity. The combination of "antigen screening + PCR diagnosis" can detect the virus and block the transmission chain more quickly and accurately.
DaAn Gene's COVID-19 saliva PCR test kit is designed for the specific identification of ORF1ab and N gene in Sars-Cov-2 genome. Detection range covers wild type 2019-nCoV and its variants to avoid missed and false detection.
Fast: Faster to get result than conventional reagent
Safe: Use of UDG enzyme to prevent carry-over contamination
Endogenous internal control: Human housekeeping gene RNase P
Simple: Use of Nucleic Acid Release and Preservation Kit to release RNA without using extraction system
>> Get Your COVID-19 PCR Test Result in 35 min
DaAn Gene’s COVID-19 Antigen Rapid Testing Kit (Colloidal Gold) has good clinical performance with high sensitivity and specificity which above the WHO recommended standard. People can get the test result in 15 minutes and significantly improve the diagnostic efficiency.
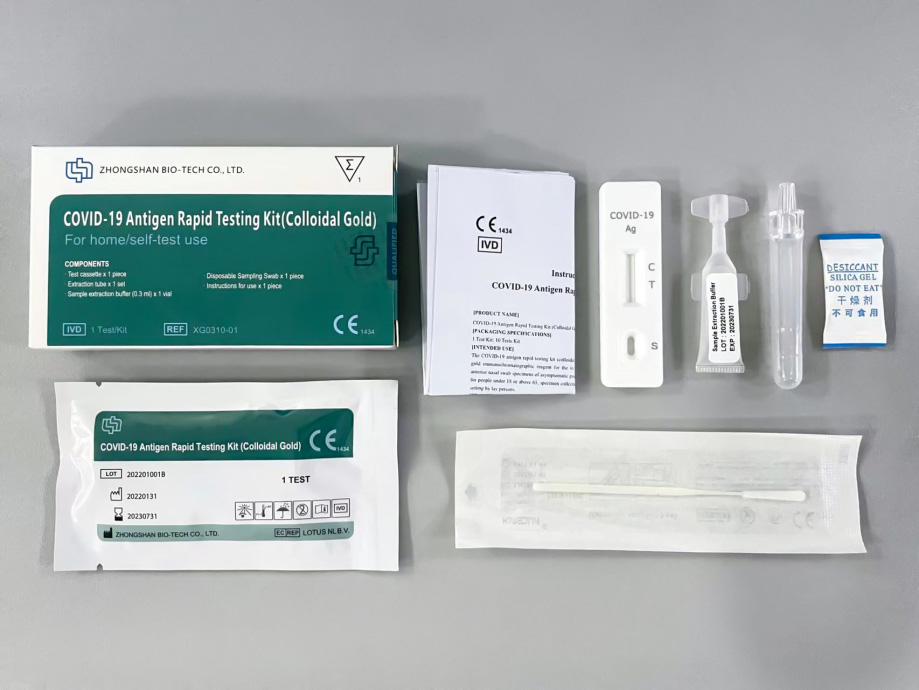
◆ User-friendly: Easy operation
◆ Fast: Get accurate result in 15 minutes
◆ Convenient: Stored at room temperature
◆ High performance: Sensitivity: 84.07% Specificity: 100% (WHO recommended sensitivity≥80% and specificity≥97% )
>> Learn More on COVID-19 Rapid Test Kit