As nucleic acid-based technology has enabled more precise quantification of HBV DNA detection, hepatitis B virus PCR detection assay is vital for the evaluation of the hepatitis b development stage and efficacy of antiviral treatment. In this article, we discuss the importance of the HBV DNA quantitative PCR test in assessing HBV infectivity and prescribing antivirus treatment for hepatitis b.
Chronic hepatitis B is a dynamic disease, patients may experience several disease phases from disease activity to inactivity. Hepatitis B virus (HBV) infection develops through several stages. The immune activation phase of chronic hepatitis B is characterized by moderate to high levels of HBV replication and infectivity. While an inactive carrier state, marked by low levels of HBV replication and infectivity. The Hepatitis B virus (HBV) DNA quantitative test can help evaluate the infection phases and infectivity of the disease, which can provide better treatment to the patient.
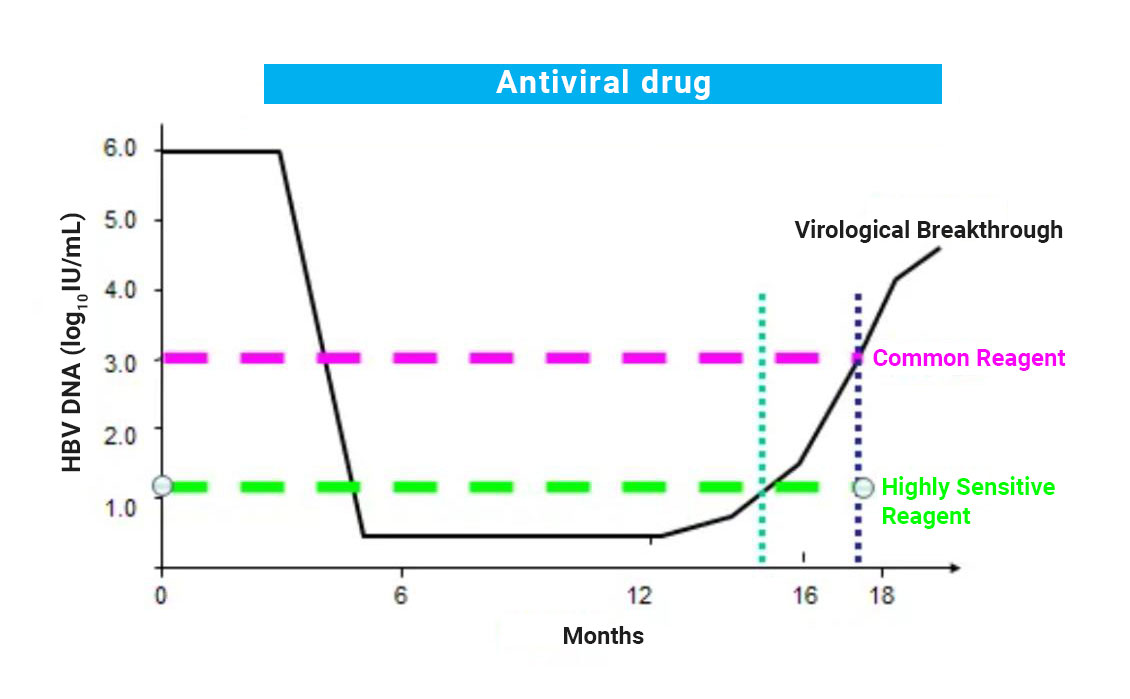
Before prescribing hepatitis b antiviral treatment, quantitative detection of HBV DNA should conduct to confirm the level of the virus, alanine aminotransferase (ALT), and the severity of the liver disease.
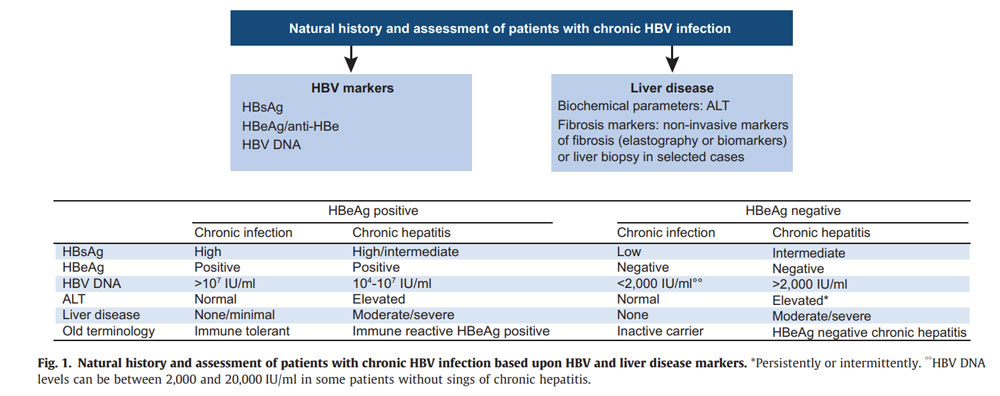
Source: https://easl.eu/wp-content/uploads/2018/10/HepB-English-report.pdf
Antiviral therapy for hepatitis B is to reduce the risks of long-term complications such as cirrhosis and HCC. Since the HBV replication and elevation of ALT levels indicate a critical risk of disease progression. Conducting antiviral therapy is aim to decrease the HBV viral load and the replication ability of the virus, so HBV DNA detection is important during hepatitis B antiviral therapy because it can evaluate the possibility of antiviral resistance.
Precise diagnosis is crucial to stop the progression of chronic hepatitis b, HBV DNA quantitative pcr test plays a significant part in measuring HBV infectivity and prescribing antivirus treatment for hepatitis b. Daan Gene’s HBV PCR test kit has obtained CE List A approval, performing high sensitivity and specificity in HBV DNA quantitative test.
Features of DaAn Gene HBV Test Kit
Wide liner range: 20 IU/mL-1.0×109 IU/mL.
Internal control: monitor the whole extraction process.
Reliable: UNG enzyme to prevent contamination.
High Sensitivity: LOD 10 IU/mL
Genotype: Cover HBV genotype A-G
>> Learn More about DaAn Gene Hepatitis B Test Kit
Reference:
1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2095015/
2. https://atm.amegroups.com/article/view/11691/html
3. Rotman Y, Brown TA, Hoofnagle JH. Evaluation of the patient with hepatitis B. Hepatology. 2009 May;49(5 Suppl):S22-7. doi: 10.1002/hep.22976. PMID: 19399815; PMCID: PMC2881483.
4. https://easl.eu/wp-content/uploads/2018/10/HepB-English-report.pdf
5. https://easl.eu/wp-content/uploads/2018/10/HepB-English-report.pdf




